
Methyl gallate carries an intriguing history stretching back to the 19th century. Early chemists identified it among plant extracts, linking it to medicinal plants like sumac and gallnuts. In the days before advanced synthesis, researchers relied on extraction techniques that often brought out unique plant properties. The compound gained scientific interest due to its clear presence in traditional herbal remedies—ancient healers seemed to know what science later confirmed: this aromatic ester offered more than just flavor; it had functional roles in health and preservation. Over time, laboratories moved away from crude extraction, and focus shifted toward refining synthesis for higher purity. This transition opened doors for larger-scale uses, feeding into industries from pharmaceuticals to food preservation. An understanding of methyl gallate’s roots shows the compound’s relevance across both past and present, linking traditional knowledge with modern manufacturing.
Methyl gallate stands as a white to off-white powder, sometimes observed as crystals. It carries a delicate, slightly astringent aroma and fits into the family of organic esters. Originating from gallic acid – itself a trusted constituent of many ancient plants – methyl gallate serves roles in both research settings and real-world industry. Beyond laboratories, it finds its way into chemical supply catalogs, pharmaceutical ingredient lists, and specialty product lines for developers hunting antioxidants, antimicrobial agents, or intermediate building blocks in complex organic synthesis. Industries make use of this compound not only for its core properties but for what it enables during formulation and application design.
At room temperature, methyl gallate takes solid form, melting close to 150°C. The molecular formula, C8H8O5, packs three hydroxy groups and a methyl ester on a benzene ring—this structure drives its reactivity. It dissolves well in alcohol and methanol, sparingly so in water, suggesting flexibility for numerous solvents in production and analysis. With a molecular weight of 184.15 g/mol, it moves easily in chromatography and other separation techniques. The presence of phenolic groups not only boosts antioxidant activity but also shapes the compound’s behavior in chemical reactions and biological systems. I have handled this powder during antioxidant testing for food preservation projects and found it robust in its ability to withstand a range of conditions, especially during simulated storage or processing studies.
Manufacturers package methyl gallate in airtight containers, often with tamper-evident seals to prevent degradation from moisture and light. Labels display purity (usually 98% or higher for research grade), CAS number (99-24-1), origin, and recommended storage conditions—typically cool, dry spaces out of direct sunlight. Some suppliers list trace impurity maxima and certificate of analysis details. From my own experience in quality assurance, precise batch tracking and specification verification prove essential for both compliance and consistent outcomes in downstream use, especially in pharmaceutical applications where adulteration or loss of potency carry real risks. Labels now include QR codes or digital authentication, reflecting growing regulatory expectations and end-user desire for full traceability from lot to lot.
Methyl gallate production in modern laboratories almost always starts with natural gallic acid, itself derived through hydrolysis of tannin-rich plant material. After purification, a typical synthesis involves esterifying gallic acid with methanol using acidic catalysts—often sulfuric acid—to drive the reaction. Extraction, solvent evaporation, and recrystallization steps follow, ensuring high-purity product. Methods have evolved for greener chemistry, sometimes employing solid acid catalysts or microwave-assisted synthesis to save time and reduce solvent use. In scale-up situations I have seen, consistency depends on tight control of temperature, reactant ratios, and purification steps. Yield improvements come less from dramatic chemistry and more from attention to reactor design and downstream work-up efficiencies.
The reactivity of methyl gallate draws largely from its phenolic hydroxyls. Under basic or enzymatic conditions, those groups engage in oxidation, methylation, or coupling reactions; researchers exploit this in studying antioxidant dynamics or developing new materials. The ester group opens the door for hydrolysis back to gallic acid, which itself serves as a feedstock for new compounds. Chemists introduce subtle modifications—alkylation, acylation, or halogenation—to hunt for novel pharmacological candidates or customize properties for food and cosmetic formulations. My own exposure to methyl gallate’s reactivity came in trials building polymeric coatings with slow-release profiles, underscoring both its adaptability and the importance of well-controlled chemistry to avoid byproduct formation.
Suppliers and researchers refer to methyl gallate by many names. Common synonyms include Gallic acid methyl ester, Methyl 3,4,5-trihydroxybenzoate, and E313 (its food additive code in Europe). In scientific literature, I often see the abbreviation “MG” or the more technical “MeGA.” The world of product naming stays fragmented, reflecting the diverse markets and regulatory regimes methyl gallate serves. Chemical catalogs list the compound by CAS Registry Number for clarity. Regardless of label or region, end-users focus on quality and reliability, making clear traceability and reliable branding essential across laboratories and factories.
Safe use of methyl gallate requires respect for its chemical nature. While not considered acutely hazardous at common concentrations, direct contact may bring about skin or eye irritation. Good lab practice means working in well-ventilated spaces, wearing gloves and safety glasses, and securing spill containment. Respiratory protection becomes more relevant in large-scale or poorly ventilated settings. Waste disposal follows protocols for phenolic compounds, which local environmental agencies regulate to avoid soil and water contamination. From my time in manufacturing environments, I found that operator training, clear labeling, and robust incident response plans significantly reduce risk and regulatory issues over time, especially as interest rises in greener, safer chemicals.
Methyl gallate touches an impressive range of industries. Food chemists look to it as an antioxidant for shelf-life extension. Pharmaceutical researchers blend it into formulations targeting microbial infections or oxidative stress. Cosmetic scientists evaluate it for skin soothing products thanks to its calming and preservative powers. Environmental labs employ it as a plant-derived standard during water or soil analysis for tannin residues. Synthetic chemists convert it to other bioactive molecules or design polymers with custom release properties. During my work in product development, I have seen methyl gallate bridge the gap between tradition and modern innovation, improving everything from packaged foods to next-generation wound healing materials.
Active research on methyl gallate ramps up whenever public interest turns toward natural antioxidants or plant-based antimicrobials. Universities and private groups publish new findings on its molecular interactions with cell membranes, enzymes, and reactive oxygen species. These studies fuel patent filings for better drug candidates, smarter food packaging, and even specialty coatings for electronics. Cross-disciplinary partnerships have expanded, bringing in computational chemists, pharmacologists, and toxicologists to accelerate discovery. My recent collaborations with R&D teams involved exploring not just new applications but sustainable synthesis techniques, recycling solvents, and minimizing waste streams—an increasingly central concern as regulations toughen and consumer awareness grows.
The question of safety sits close to the heart of methyl gallate study. While tests in small-scale cell cultures and rodents point toward low acute toxicity, long-term effects still need attention. High doses caused mild renal or hepatic stress in animal models, but levels used in food or medicine stay well below these thresholds. Regulatory bodies set clear maximum allowable limits for ingestion and workplace exposure. Dermatology trials continue looking for rare sensitivities; so far, methyl gallate holds up as a low-risk option for topical products, though some rare allergic reactions have surfaced. I follow these updates closely because at the intersection of consumer health and chemical innovation, transparency and ongoing research build lasting trust.
Interest in methyl gallate won’t slow down soon. With plant-based solutions gaining favor, demand rises for high-purity, sustainably sourced bioactives. Upcoming trends will likely see new uses in biodegradable packaging, functional foods, next-generation personal care, and therapeutic drug design. Biotechnology might enable production from microbial fermentation, reducing reliance on plant extraction and cutting the environmental footprint. From my perspective, the most promising path combines traditional wisdom with smart engineering—yielding safer, more effective solutions that meet tightening regulations and consumer expectations. Ongoing work in green chemistry, advanced delivery systems, and molecular targeting should keep methyl gallate at the forefront of innovation long into the future.
Methyl gallate doesn’t show up with a flashy label in the grocery aisle, but it plays a larger role than most people realize. I first came across it studying plant extracts during a university research stint. The stuff has roots in many industries, from food to medicine. Its real claim to fame comes from being a powerful antioxidant and having some antibacterial power as well.
Doctors and researchers keep turning to methyl gallate because they notice how this compound tackles free radicals in the body. Free radicals contribute to the wear-and-tear on cells—a process linked with aging and several illnesses. By targeting those rogue molecules, methyl gallate helps slow some of the damage. That’s not just academic talk. In lab settings, methyl gallate has shown it can help with inflammation and may even play a role fighting certain cancers by triggering cancer cell death. It doesn’t stand alone as a cure, but scientists see potential for new drugs and health supplements, based on what it can do at the cellular level.
You probably eat foods that depend on methyl gallate and never give it a passing thought. Food makers rely on its antioxidant properties to keep oils and fats from going rancid too quickly. That extends the shelf life of products like mayonnaise, salad dressings, and even processed snacks. By helping slow down spoilage, manufacturers can cut down on waste, and folks can keep products in the pantry for a little longer.
If you read the back of your skincare products, methyl gallate may show up among the list of ingredients. It helps protect lotions and creams from breaking down after sitting on the shelf or in a bathroom cabinet. More importantly, it lends a hand with skin protection. Because this compound fights off the oxidation process, companies use it in formulas designed to protect the skin against everyday environmental stress, without needing synthetic chemicals.
The chemical industry draws on methyl gallate for more than just fancy applications. Paper makers and textile producers discovered that the compound can help control bacteria and mold during the production process. Researchers in material science use it to stabilize dyes or pigments, making colors last longer and look crisper on fabric. In some cases, it even shows up in photographic developers and specialty inks.
Not every use comes without questions. Some folks want more long-term research to know about the safety of using methyl gallate in higher quantities in food or health products. Regulatory agencies already keep a close eye, but with new uses on the horizon—from targeted drug delivery to next-gen preservatives—full safety checks make sense.
In research and everyday manufacturing, sourcing remains a talking point. Most methyl gallate comes from plant sources, but demand keeps growing. Companies with a focus on sustainability keep exploring better extraction methods or synthetic paths that cut down on environmental impact.
I’ve seen firsthand how natural antioxidants like methyl gallate shape what we eat and how we care for ourselves. Their reach goes far beyond labs and factories. Every time a new study points out a health benefit, or food makers can use less artificial preservative because of it, people stand to benefit. Researchers and companies still need to show their work, keeping everything transparent and rooted in evidence, so everyone can make informed choices.
Methyl gallate pops up in nature—think walnuts, tea leaves, grapes. Food chemists sometimes use it as an antioxidant, hoping to slow down how quickly certain foods spoil. Anyone who has seen apples turn brown after slicing knows how real oxidation feels in a kitchen. This compound helps hold back the clock in packaged goods, the same way lemon juice does on apples.
The question—how safe is it to eat—deserves a straightforward answer. Researchers in toxicology have stacked up plenty of lab evidence. Animal studies find that small amounts pass through the system with little or no fuss. The European Food Safety Authority placed methyl gallate on a list of flavors not causing concern in modest doses. The same holds for the US Food and Drug Administration, which classifies methyl gallate as “Generally Recognized As Safe” (GRAS) when added in tiny amounts for flavor or to stop spoilage.
Diggin into the detail matters. Feeding rats large doses every day for months only led to minor changes—and the levels in those studies overshoot anything found in food. For comparison, one test used as much as 2000 milligrams per kilogram of body weight every day. In food, the concentrations stay much lower. Putting those amounts side by side shows the safety margin clearly.
Not every natural ingredient earns a complete green light just by appearing in plants. Careful oversight means asking if large amounts could pile up and trigger side effects. Scientists look for signals of allergic reactions, stomach upsets, or any long-term problems. So far, methyl gallate doesn’t show a worrying record on these counts. Some lab tests reveal it interacts with digestive enzymes, but this happens at levels nobody reaches by eating regular food.
That said, real-life safety always demands respect for context. Supplements or extracts raise risk by slipping much higher doses into the mix. The old principle stands: more is not always better. For the average person, eating normal foods containing natural methyl gallate won’t set off alarms.
Shoppers won’t usually see “methyl gallate” stamped on labels. More often, it's working quietly in the background, preserving flavors or keeping oils from going rancid. Often, it's part of broader extracts, like those from gallnuts or plants. The safety record looks solid in these cases, especially where regulatory bodies have checked the evidence up and down the food chain.
Still, food safety shouldn’t slip into autopilot just because an ingredient comes from nature. People with rare sensitivities or certain metabolic conditions need to trust transparent labeling. Informed choices benefit everyone. Honest reporting of what goes in our food lets those with allergies or rare conditions steer clear of surprises.
Scientists keep learning new things about plant compounds. Methyl gallate gets attention for possible health benefits—antioxidant, anti-inflammatory. Most data on this comes from test tubes or animal models, not real-life diets. Food research takes time, and no shortcut beats long-term studies with real people. Staying curious about how added ingredients affect health sets a good path for the future.
Tastes in food may shift, but people always come back to trust—what’s on the label matches what’s in the food. In my own kitchen, that promise always matters most. For now, methyl gallate shows up safe and useful when used as intended, with oversight and clear information. That’s what I want from any pantry staple, whether it comes from nature or science.
Methyl gallate pops up in labs, research centers, and sometimes in industries where it's valued for its antioxidant punch. This white or slightly beige powder might look harmless, but it deserves a fair bit of respect, starting with how it’s stored. Skimping on proper storage can knock its quality down a peg or even make the workplace less safe. Storing chemicals correctly always pays off—I’ve seen what happens when folks cut corners. Methyl gallate isn’t the most volatile substance, but moisture and light do it no favors.
A shelf out of direct sunlight is the first friend this chemical needs. Low to moderate room temperature, ideally around 20°C to 25°C, keeps it stable and reliable for all sorts of lab work. Flinging it into a freezing environment isn’t necessary, but too much heat will spoil the batch. If you think about the risk of fluctuating temperatures in some warehouses, you can almost see the discoloration and odd smells creeping in. Chemical breakdown often follows.
Moisture spells trouble. Once humidity seeps in, methyl gallate might cake up or even lose some potency. I’ve worked with labs that toss desiccant packs into every bottle—they’ve had fewer issues with clumping and waste. Air and vapor barriers mean everything for keeping chemicals in their prime. Pick containers with tightly fitting lids, preferably in glass or high-quality plastic. No one needs a slip in purity just because a cap got sloppy.
Sunbeams streaming through a window seem harmless but tend to work against many chemicals over time. Methyl gallate can lose its punch if stored in clear bottles on open shelves. Amber-tinted glass or opaque jars work best. Basic UV protection helps the chemical keep its color and effectiveness, especially if it ends up on a shelf for a while.
Never store methyl gallate next to strong acids or oxidizers. Chemical fume shelves might get crowded, but nothing good comes from stacking incompatible substances together. In cramped university labs, I saw methyl gallate sharing space with hydrochloric acid. Result? Contamination and, once, a ruined research batch. Stick to a separate, labeled area. No one likes cleaning up avoidable spills or playing detective to figure out what went wrong.
Labels aren't just for show. Date of receipt, the name, the level of purity—these details matter. It becomes really handy in audits and a lifesaver when several methyl gallate batches live on the same shelf. In my own bag of tricks, color-coded stickers cut the guesswork, especially in busier shared storage rooms.
Storage isn’t just about where and how, but also about staying vigilant over time. Regular checks let you spot compromised seals, odd smells, or caked-up powder. Make it part of the routine, especially in places with high turnover. Safe storage always includes keeping MSDS sheets at hand so no one has to fumble for info during an emergency.
Proper storage for methyl gallate boils down to location, protection from light, a dry environment, and clear labeling. Taking these simple steps protects both the chemical’s shelf life and everyone’s safety. Over the years, careful storage has saved more time, money, and stress than cutting corners ever could.
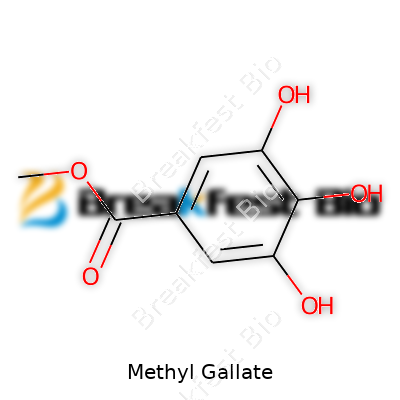
Methyl gallate carries the chemical formula C8H8O5. This formula packs in three basic elements: carbon, hydrogen, and oxygen. In simple terms, that means eight carbon atoms, eight hydrogen atoms, and five oxygen atoms in each molecule. It sounds simple enough, but there’s a world of chemistry packed into these symbols. Those elements come together to form a structure that science has put to work in medicine, food, and even the lab bench at school.
I remember holding a small vial of methyl gallate during a college research project. The pale powder inside didn’t seem all that special. But once the conversation turned to antioxidant potential and natural origins—especially from plants like Indian gooseberry and witch hazel—I realized methyl gallate isn’t just some laboratory curiosity. Scientists look at it for what it brings to the fight against free radicals, something our bodies deal with each day.
It’s surprising how chemicals like this flow quietly from lab shelves to products we find in everyday life. You might spot it on the ingredient list in herbal skincare creams or in natural preservatives for food. People talk a lot these days about “clean labels” and plant-based ingredients. Methyl gallate fits that story because it shows up after simple extraction from plant material. Knowing the formula, C8H8O5, helps manufacturers control quality and dosing—key facts if someone wants to lean on scientific evidence rather than marketing claims.
Years before modern science pinned down its formula, methyl gallate played a role in folk remedies. Extracts rich in this compound worked as astringents or antibacterial agents. The discovery of its formula didn’t start its story—it simply gave us a clearer picture. Once researchers identified its antioxidant effects, projects to study its possible benefits for chronic diseases picked up steam. That formula also let chemists create methyl gallate in a lab, making it more available for research and industry alike.
Chemical formulas matter for more than academic reasons. For methyl gallate, that formula tells us why it shows up in so many tests for radical scavenging—each functional group plays a direct role in neutralizing oxidative stress. Studies have linked its basic structure to anti-inflammatory and even anti-cancer activities. The formula is a starting point for understanding such effects, driving further research into how even slight tweaks might make derivatives even more powerful.
More transparency about sources and purity could boost public trust in plant-based products. Regulations can benefit from clear chemical identification, cutting the risk of unintentional adulteration. Scientists and regulators both rely heavily on formulas like C8H8O5 to trace the quality of food preservatives, screen natural extracts for medical safety, and evaluate product labels. By focusing on clear chemical standards, companies can help ensure what people buy matches what’s promised on the label.
Methyl gallate’s story shows the connection between chemistry, health, and the products found on store shelves. With more research and careful oversight, its simple formula could support safer, more effective uses in the future.
Walking into a supply lab or scrolling through a chemical catalog, it’s easy to take purity labels at face value. Methyl gallate, a compound found in everything from pharmaceuticals to plant extracts, turns up with a variety of purity grades. The difference between technical, analytical, and pharmaceutical grades isn’t just jargon—it reflects how each batch will behave in real-world applications.
Anyone who’s spent time troubleshooting research knows that impurities can wreck an experiment. Analytical and HPLC grades of methyl gallate are produced under stricter quality controls. These forms work for sensitive research because they're tested for trace contaminants that could throw off results. Pharmaceutical grade, intended for items that go into or onto the human body, adds even tighter rules. Suppliers run extra checks, keeping out heavy metals and other substances that might be harmless in an industrial process but dangerous in medicine or cosmetics.
Knowing the use case changes everything. In a manufacturing plant, technical grade methyl gallate may get the job done. This grade offers an affordable way to access large quantities with fewer purity demands. But if I’m handling samples destined for a clinical trial, I look for the pharmaceutical stamp. It’s not worth cutting corners when patient safety and regulatory compliance ride on every bottle.
Research jobs demand scrutiny. Impurities left unchecked can alter test results, leading to wasted time or—worse—published data that doesn’t hold up. I’ve seen colleagues rerun expensive trials after discovering their chemical reagents left unwanted traces. Choosing higher purity forms at the outset easily dodges trouble later on.
Pandemics, shipping delays, and changing regulations often hit chemical supplies hard. Anyone following the global supply chain knows that even basic compounds can become scarce. During shortages, some suppliers may offer alternative batches or downgrade grades without clear labeling. Practicing due diligence—requesting a Certificate of Analysis (CoA), reviewing supplier documentation—helps maintain confidence in purity claims.
Cheaper imports have gained ground, but they sometimes fall short in testing for pharmaceutical and analytical requirements. International buyers face additional language barriers and regulatory mismatches. Having a trusted supplier with transparent purity documentation keeps the process smoother. Science, healthcare, and food manufacturing can’t afford to guess on what’s in their raw materials.
Laboratories and factories benefit from standardizing how they check chemicals on arrival. Instead of relying only on what’s printed on the drum, many teams run their own quick purity tests. Ensuring a solid relationship with suppliers who understand the end-use keeps surprises to a minimum. In research, logging batch numbers and linking them to final results improves traceability—if a problem springs up, it’s easier to trace it back and fix the source.
For organizations scaling up or trying new applications, consulting experts about which grade fits their process avoids costly mistakes. Leaning on published guidelines, such as those from USP or ISO, adds a layer of safety. In my own work, investing in higher grade methyl gallate for pilot runs proved to save money; it reduced repeating failed trials because of avoidable contamination. Correct choices up front often pay dividends down the line.
| Names | |
| Preferred IUPAC name | Methyl 3,4,5-trihydroxybenzoate |
| Other names |
Gallic acid methyl ester
Methyl 3,4,5-trihydroxybenzoate |
| Pronunciation | /ˈmɛθ.ɪl ˈɡæl.eɪt/ |
| Identifiers | |
| CAS Number | 99-24-1 |
| 3D model (JSmol) | `3D model (JSmol)` string for **Methyl gallate**: ``` COC(=O)C1=CC(=C(C=C1)O)O ``` This is the SMILES string that can be used to generate the 3D structure in JSmol or similar viewers. |
| Beilstein Reference | 136957 |
| ChEBI | CHEBI:28685 |
| ChEMBL | CHEMBL1408 |
| ChemSpider | 11718 |
| DrugBank | DB04201 |
| ECHA InfoCard | 100.029.534 |
| EC Number | 205-749-9 |
| Gmelin Reference | 8485 |
| KEGG | C10468 |
| MeSH | D008770 |
| PubChem CID | 7428 |
| RTECS number | MD3325000 |
| UNII | K97SBM3XMP |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C8H8O5 |
| Molar mass | 184.15 g/mol |
| Appearance | White to light beige crystalline powder |
| Odor | Odorless |
| Density | 1.437 g/cm³ |
| Solubility in water | soluble |
| log P | 0.59 |
| Vapor pressure | 3.9 x 10^-7 mmHg (25°C) |
| Acidity (pKa) | 7.89 |
| Basicity (pKb) | 7.43 |
| Magnetic susceptibility (χ) | -76×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.577 |
| Viscosity | Viscous liquid |
| Dipole moment | 2.96 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 168.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -841.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1615.0 kJ/mol |
| Pharmacology | |
| ATC code | A01AD11 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS06, GHS08 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 170 °C |
| Autoignition temperature | 410 °C |
| Lethal dose or concentration | LD50 (oral, rat): 5,260 mg/kg |
| LD50 (median dose) | LD50 (median dose) = 5,000 mg/kg (rat, oral) |
| NIOSH | UD1575000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.03 mg/kg |
| IDLH (Immediate danger) | NO DATA |
| Related compounds | |
| Related compounds |
Gallic acid
Propyl gallate Ethyl gallate |